BPI Biotech holds Writeshop for Procedures Manual, Risk Assessment Database of GM Crops
By: Paulyne Nathalie Ordillo
Posted September 1, 2021
The Bureau of Plant Industry-Biotechnology Office in collaboration with the Department of Agriculture-Biotechnology Program Office held a two-day online writeshop for the Manual of Procedures for Joint Department Circular No. 1 Series of 2016 and Risk Assessment Database for GM crops last August 31- September 1, 2021, via Zoom.
Representatives from the BPI-Biotechnology Core Team (BCT), BPI-Food Safety Risk Assessment Team (FSRAT) and Bureau of Animal Industry-Biotech Team (BAI) attended the said activity, which aims to draft and finalize the manual of procedures and risk assessment database for the application of biosafety permits of genetically modified crops for field trial, commercial propagation, and direct use.
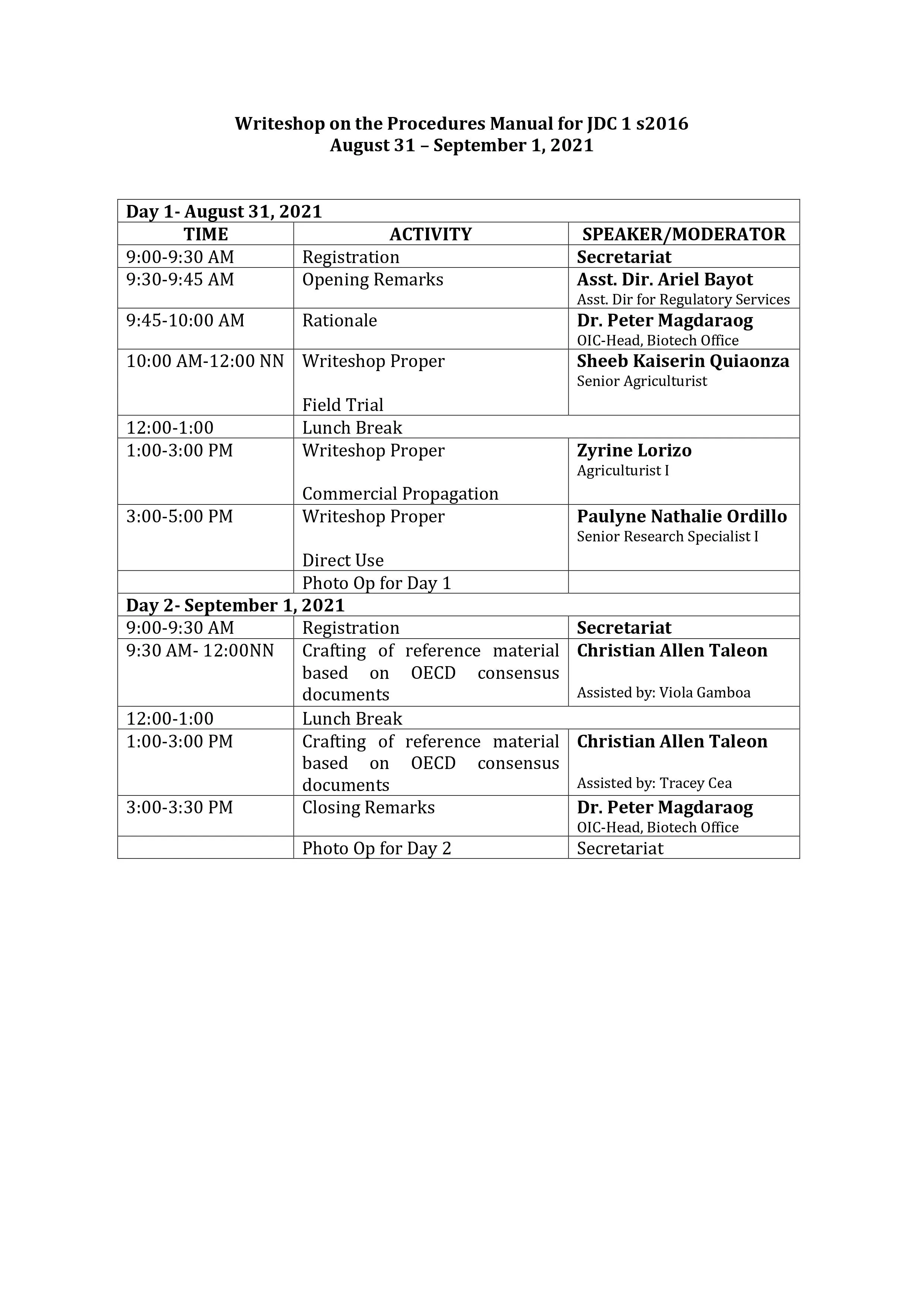
Dr. Peter Magdaraog, OIC-Head of the BPI Biotechnology Office delivered his opening message and gave the rationale of the online writeshop. For Day 1, Ms. Sheeb Kaiserin Quiaonza, Senior Agriculturist and Head of the Biotechnology Secretariat facilitated the review and revision of the manual of procedures for the application of biosafety permit for field trial. This was followed by a discussion on the procedures for commercial propagation and direct use, facilitated by Ms. Zyrine Lorizo, Agriculturist I, and Ms. Paulyne Nathalie Ordillo, Science Research Specialist I, respectively.
For the second day of the activity, Mr. Christian Allen Taleon, Agriculturist II and Chair of the BPI-FSRAT presented the risk assessment database and facilitated the crafting of the reference material based on OECD consensus documents. The members of BPI-FSRAT initiated the creation of a database for the most common information for host organisms, proteins, and their respective donor organisms, as well as the most common genes used in genetic transformation. For the writeshop proper, the BAI Biotech Team made valuable inputs to the GM crops database based on the information obtained from the OECD document. Their output was presented and reviewed during the afternoon session. Members of BCT and BPI-FSRAT also presented their entries to the protein and donor organism database. They were assisted by Ms. Viola Katherine Gamboa, Agricultural Technician II, and Ms. Zyrine Lorizo, Agriculturist I, both members of the Biotechnology Secretariat.
Mr. Ariel Bayot, Assistant Director for Regulatory Services, gave his closing message and commended the group for participating in the two-day writeshop. He also stressed the importance of the development and implementation of an effective biosafety regulatory framework, and the essential role of risk assessment in modern agriculture.
The writeshop ended with a virtual photo op with the participants facilitated by the host of the event, Ms. Tracey Mae Cea, Senior Administrative Officer II and member of the Biotechnology Secretariat.
Overall, the activity was a success, and has resulted in two working documents that will greatly aid in the conduct of biosafety regulations for GM crops in the Philippines.