BPI Biotechnology Office Writeshop and Policy Review of Issuances Under DOST-DA-DENR-DOH-DILG Joint Department Circular No.1 s2021
By: Ericka Joy U. Ancayan
Event August 2, 2022
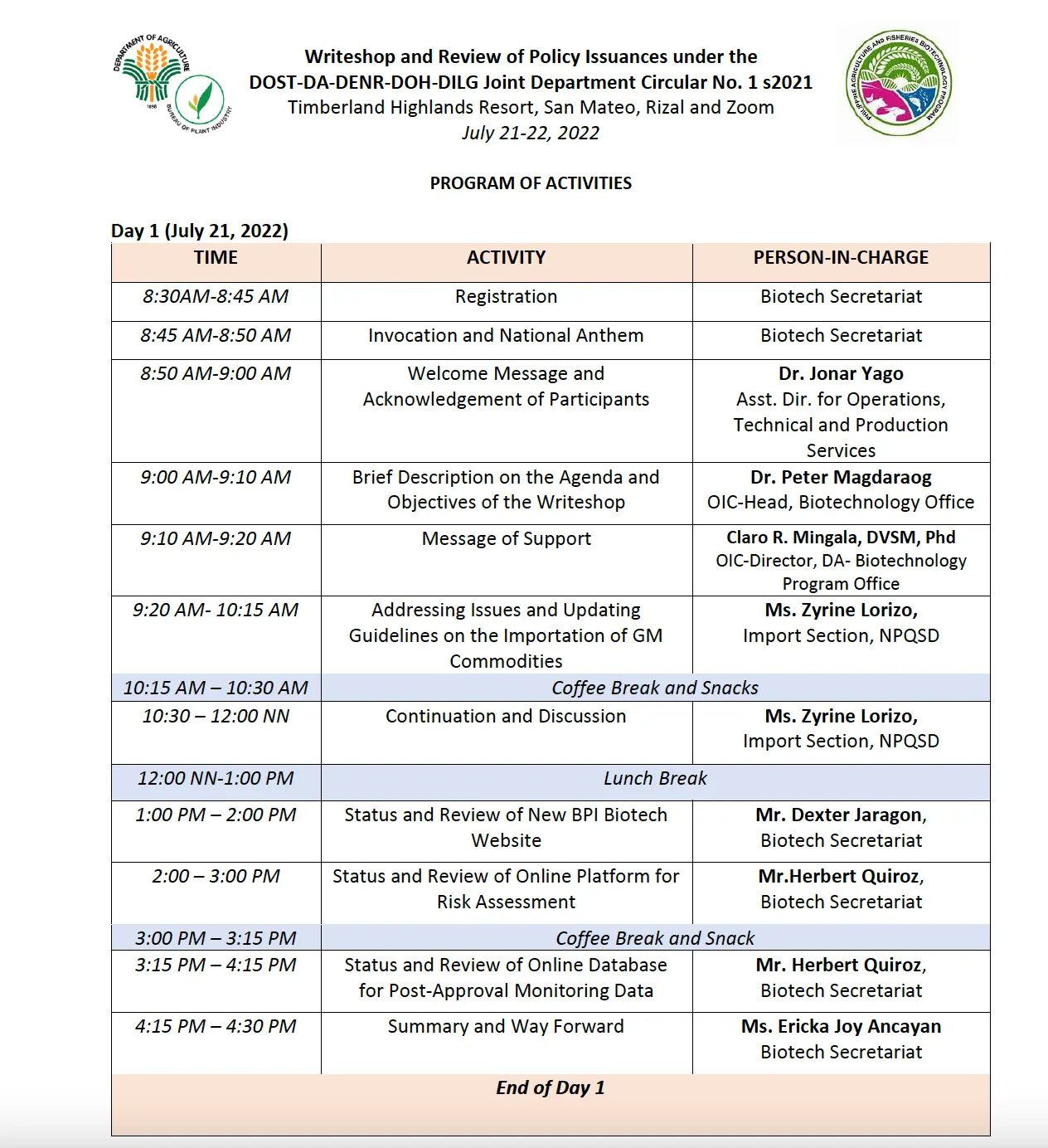
The Bureau of Plant Industry (BPI) Biotechnology Office in partnership with the Department of Agriculture Biotechnology Program Office (DA-BPO) conducted a Writeshop and Review of Policy Issuances under the DOST-DA-DENR-DOH-DILG Joint Department Circular No. 1, series of 2021 (JDC No.1, s2021) on July 21-22, 2022 at Timberland Resort, San Mateo, Rizal.
The two-day writeshop aimed to review and finalize the supplemental guidelines for JDC No.1, s2021, and provide technology developers a clear guidance on their roles and responsibilities involving genetically modified (GM) crops regulations. Members of the Biotech Core Team-Joint Department Circular (BCT-JDC), BCT-Post Approval Monitoring Group (BCT-PAMG), and DA policy offices joined the activity through a hybrid setup via Zoom and face-to-face attendance.
Dr. Jonar I. Yago, Assistant Director for Operations and Technical and Production Services formally opened the first day program through his welcoming remarks, highlighting the goal of upscaling the activities and programs of the Biotechnology Office. He also emphasized the importance of a harmonized guideline for GM regulations. Moreover, Dr. Peter Magdaraog, OIC-Head of the Biotechnology Office, presented the agenda of the writeshop and shared that the activity is one of the target outputs of the recently approved BAR project entitled “Upgrading the Biosafety System Through Enhanced Information Accessibility, and More Efficient and Streamlined Regulatory Process.”
During the first day sessions, Ms. Zyrine Lorizo, Agriculturist II from National Plant Quarantine Services Division (NPQSD) facilitated the discussion on addressing the issues and updating the guidelines of GM commodities importation. The writeshop also tackled the ongoing development of the new BPI Biotech websites and post approval monitoring database presented by Mr. Dexter Jarogon and Mr. Herbert Quiroz, Biotechnology Secretariat members.
During the seminar, Mr. Reimond Corona, Project Senior Technical Specialist of the National Committee on Biosafety of the Philippines (NCBP) reviewed the JDC No. 1, s2021. This was followed by the lecture of Dr. Subray Hegde, Director of the Biotechnology Risk Analysis Programs of the Biotechnology Regulatory Services of APHIS USDA, shared a regulator’s experience, including field trial data from other countries in safety assessments.
Meanwhile, the second day schedule entirely focused on polishing the supplemental guidelines under JDC No.1, s2021, and issued Memorandum Circulars. The roles and responsibilities of technology developers on the application of biosafety permits, post-permit issuance compliance, grounds and procedure for revocation, and miscellaneous concerns were reviewed and finalized throughout the sessions. Moving forward, a consultative meeting will be held to discuss the writeshop and policy review outputs with the stakeholders.
Lastly, Dr. Peter M. Magdaraog delivered his closing remarks and appreciated the valuable contribution of the participants to further improve the policies and guidelines. The event was hosted by Ms. Viola Katherine Gamboa and Ms. Tracey Mae Cea, both members of the Biotechnology Secretariat.